Soil pH is the measure of a soil's acidity or alkalinity. It is a critical factor in determining the health and productivity of plants including the oil palm. Soil pH plays an important role in regulating nutrient availability to plants, influencing various biological and chemical processes within the soil. As planters, understanding the relationship between soil pH and plant nutrient availability is essential to ensure that we care for our crops correctly.
Understanding Soil pH
Soil pH is measured on a scale ranging from 0 to 14, with 7 being considered neutral. Values below 7 indicate acidic soils, while values above 7 are alkaline. The pH scale is logarithmic, meaning that a change of one unit on the pH scale represents a tenfold change in acidity or alkalinity. For instance, a soil with a pH of 6 is ten times more acidic than a soil with a pH of 7.
Soil pH can affect the availability of different plant nutrients: at different pH, plant nutrients can change into different forms that can or cannot be absorbed by plants. Typically, most nutrients are adequately available to plants at neutral pH, and hence a vast majority of plants thrive on neutral soils (see Table 1).

Table 1: Soil pH and suitability for plants.
Source: Colorado State University – CMG Garden Notes #222
The oil palm is a unique plant that is able to withstand acidic soils as low as pH 4.0. This is fortunate given that most tropical soils such as in Malaysia and Indonesia are more acidic compared to soils in temperate countries. Nevertheless, very acidic conditions such as in acid sulfate soils (soil pH < 3.5) are harmful to plants as they can damage roots, inhibit root growth and elongation, and inhibit the uptake of many plant nutrients.
Plant growth relies on a variety of essential nutrients, including macronutrients (nitrogen, phosphorus, and potassium) and micronutrients (iron, zinc, manganese, etc.). The availability of these nutrients in the soil is closely linked to its pH. Soil pH impacts nutrient availability through several mechanisms, mainly affecting the solubility and mobility of these nutrients (see Fig. 1).
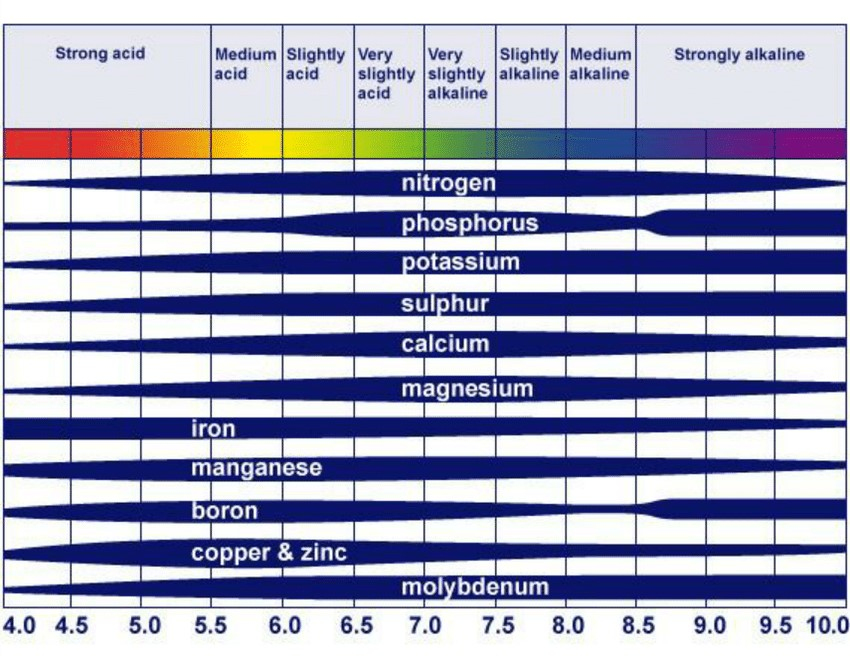

Fig. 1: Availability of different plant nutrients with respect to soil pH.
1. Macronutrients
- Nitrogen (N): Nitrogen is essential for plant growth, and its availability is influenced by soil pH. In acidic soils (pH < 6), the solubility of aluminum and iron increases, leading to potential toxicity issues that can hinder nitrogen uptake. On the other hand, in alkaline soils (pH > 7), the conversion of ammonium nitrogen to nitrate nitrogen is hindered, limiting the availability of nitrate nitrogen to plants. Therefore, maintaining a near-neutral pH level is crucial for optimizing nitrogen availability.
- Phosphorus (P): Phosphorus availability is highest in slightly acidic to neutral soils. Alkaline soils can reduce phosphorus availability by forming insoluble compounds with calcium. In contrast, acidic soils can make phosphorus less available by binding it to iron and aluminum. Adjusting soil pH to the optimal range (around 6.0 to 7.0) is essential to maximize phosphorus uptake.
- Potassium (K): Potassium is relatively unaffected by soil pH, as it remains soluble and available over a broad pH range. However, the pH level can still indirectly affect potassium uptake by influencing other nutrient availability and overall plant health.
2. Micronutrients
- Copper (Cu) and Zinc (Zn): Copper and zinc availability decreases in alkaline soils due to the formation of less soluble compounds. In acidic soils, these elements can become more available, but extreme acidity can lead to excessive levels that are toxic to plants. A pH range of 6.0 to 7.0 is recommended for optimal copper and zinc availability.
- Manganese (Mn): Manganese is most available to plants in slightly acidic soils. Alkaline soils can reduce manganese availability, while highly acidic soils can make it excessively available, potentially causing toxicity. Maintaining a pH level between 5.5 and 6.5 is optimal for manganese uptake.
Adjusting Soil pH
Apart from understanding the relationship between plant nutrient availability and soil pH, we should also know how to adjust the soil pH in order to achieve more favorable growing conditions for our crops:
- Lime Application: To raise soil pH (i.e., make it less acidic), the most common liming agents used in Malaysia for oil palm are limestone and ground magnesium limestone (GML). The latter contains magnesium (Mg) and may be used to improve the soil Mg content. In Indonesia, dolomite is more commonly used to amend acidic soils and provide Mg nutrition. Dosage can range from 1 to 3 kg per palm per year, depending on your agronomist’s recommendations. Liming agents should not be applied together with urea, as it can cause severe nitrogen volatilization losses.
- Bunch Ash Application: For oil palm growers with access to a palm oil mill, bunch ash is also a good way to amend acidic soils. It is also rich in potassium and contains small amounts of calcium, boron, phosphate, and other nutrients. Bunch ash is highly alkaline and caustic, so handle it with care. Apply up to 3 kg per palm, twice a year.
- Organic Matter: Incorporating organic matter such as empty fruit bunches (EFB) or animal manure into the soil can help buffer pH and maintain it in the ideal range for a wide range of plants. Organic matter acts as a natural pH stabilizer and enhances the overall health of the soil. Animal manure and EFB are also rich in nitrogen and phosphorus and can supplement chemical fertilizer use.
Soil amendments to lower soil pH (i.e. make soil more acidic) are rare in Malaysia as soils here are already acidic. However, it should also be noted that some of the chemical fertilizers we use such as ammonium sulfate, ammonium chloride, and MOP (potassium chloride) can further acidify soils over prolonged use. Therefore, there should be a system of monitoring soil pH in place.
Testing and Monitoring Soil pH
The key to successful soil pH management is regular testing and monitoring. It is recommended to send soil samples to a professional laboratory once every five years for a full analysis, while in-house monitoring can be done more frequently using soil pH testing kits. Professional testing is usually done at two different soil depths: 0 – 15 cm from the soil surface and 15 – 45 cm. Once you know your soil's pH, you can make informed decisions about making pH adjustments as necessary to suit your crop. Frequent monitoring ensures that your soil pH remains within the desired range, promoting healthy growth and high crop yields.
Conclusion
Soil pH is a fundamental factor in determining plant health and nutrient availability. It plays a pivotal role in regulating the solubility and mobility of essential nutrients, affecting the overall growth and productivity of plants. To optimize nutrient availability, it's crucial to maintain the appropriate pH level for your specific crops. Whether you need to raise or lower the pH of your soil, there are various methods available to help you achieve the desired pH range. Regular soil testing and monitoring are essential for ensuring that your plants receive the necessary nutrients and thrive in their environment. Understanding and managing soil pH is a fundamental skill for any grower looking to achieve healthy and productive crops.
